Best Management Practices for Following Sugar Beets with Corn
July 24, 2024
- Sugar beets are a non-host for arbuscular mycorrhizal fungi (AMF), and corn relies on AMF development for nutrient uptake and resiliency.
- Growers should assess the risk of corn experiencing fallow syndrome following a sugar beet crop.
- Mycorrhizal colonization and phosphorus starter fertilizer can support corn germination and avoid the growth lag associated with limited mycorrhizae and phosphorus uptake.
Mycorrhizae Benefits and Functions
Arbuscular mycorrhizal fungi (AMF) expand the ability of corn roots to acquire nutrients, especially immobile nutrients such as phosphorus, zinc, and copper. Corn root hairs are limited to acquiring nutrients and moisture from the nearest one to two millimeters of surrounding soil, but AMF hyphae of can reach 15 cm from the plant roots they feed.1 In exchange for nutrient scavenging, the host plant provides AMF with photosynthates or sugars. Mycorrhizal development helps corn stay resilient to drought and root diseases while supporting optimal growth potential. The hyphae also benefit the environment around them by improving soil structure and reducing nutrient loss.
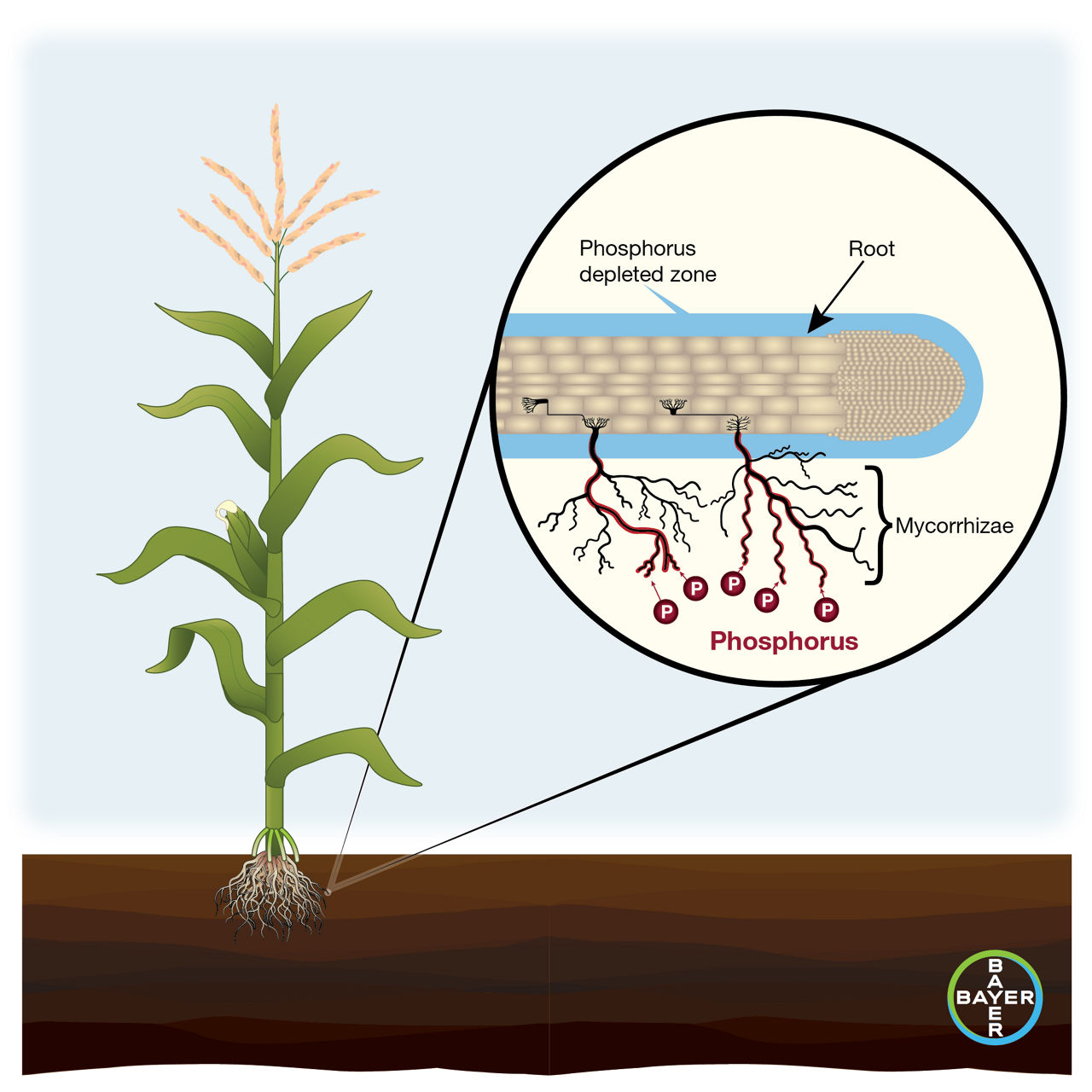
Mycorrhizae Propagules
Spores, fragments of colonized crop roots, and viable mycorrhizal hyphae act as propagules of arbuscular mycorrhizal fungi. Sugar beets are a non-host for AMF. Consequently, corn fields following sugar beets are at a greater risk for fallow syndrome due to the time necessary for the corn roots to develop symbiotic mycorrhizae colonies.

Mycorrhizae propagules act as an inoculum to form the symbiotic relationship with the next living host crop. Industry has tried to market these propagules as an inoculant in the way rhizobium has been developed to aid soybean nitrogen fixation. However, unlike with soybean rhizobium, there has been a lack of regulation and standards for mycorrhizae propagule development. A recent study across three countries discovered 84% of tested commercial inoculants did not contain viable mycorrhizae propagules.2 Inconsistent success and economic value from these products have led growers to seek other solutions to fallow syndrome in corn following sugar beet crops.
Assessing Fallow Syndrome and Increasing Arbuscular Mycorrhizal Fungi
As the concentration of phosphorus in the soil increases, the growth of arbuscular mycorrhizal fungi decreases. This is because host plant roots send signal molecules to their AMF based on the phosphorus levels in the plant tissues. In other words, corn plants in need of phosphorus would signal their roots to encourage the development mycorrhizal colonies.
Formation of mycorrhizae can be promoted by increasing plant cover with cover crops which host mycorrhizae: millet, rye, oat, clover, and vetch.3 Reducing soil disturbance and diversifying crop rotation are also recommended to augment AMF populations. Though even with low mycorrhizae populations, fields with high phosphorus levels—especially manured fields—are less likely to experience fallow syndrome after a year of sugar beets.
Starter Fertilizer and Corn Seed Treatment to Support Seedling Growth
In most scenarios, a starter fertilizer (in a 2X2 application) including phosphorus and zinc has been recommended at planting when following sugar beet with corn. However, broadcasted zinc and phosphorus have been unsuccessful for remedying fallow syndrome in corn.4 The following treatments can be used as a guide to provide enough nutrients to corn seedlings during the lag time before AMF colonization.
- Banding at a rate of 5 gal/acre of N-P-K fertilizer product 10-34-0 could be enough to prevent corn-leaf purpling associated with phosphorus deficiency.4
- A P2O5 product can be band-applied a rate of 40 lb/acre at a safe distance from rows to prevent seed damage.
- Chelated zinc should be applied on seeds.
At the seed level, BioRise® corn offering includes two components to aid phosphorus uptake and mycorrhizal colonization. BioRise® corn offering includes the on-seed application of Acceleron® B-300 seed applied technology (Penicillium bilaiae) and Acceleron® B-360 seed treatment. Penicillium bilaiae is a naturally occurring soil fungus that helps release phosphorus bound to soil. Acceleron® B-360 enhances mycorrhizal colonization. Results from 100 trials over three years showed mycorrhizal colonization increased 85.1% on corn using BioRise® corn offering compared to corn without BioRise® corn offering. Fields with cool, wet soil temperatures should be planted last to allow additional days to warm and dry to give microbial communities the right conditions to start growing.
Corn is expected to outgrow purpling symptoms at the two-leaf growth stage. Some growers have had success rescuing purpled corn at the four-leaf stage by sidedressing nitrogen and phosphorus. The additional soil surface disturbance for fertilizer incorporation, or between-row cultivation, seems to relieve cold and wet soil conditions.
Conclusion
Fallow syndrome needs to be addressed before phosphorus deficiency affects corn growth. Fields with low phosphorus levels and dormant or low numbers of AMF propagules have the greatest risk of fallow syndrome. There are no guarantees on the necessity of or economical return on remedies for fallow-syndrome. The value of nutrient cycles and microbial systems are difficult to quantify by dollar or yield volume. Engineered by nature, their worth is measured by crop resiliency.
Sources
1 Lohman, M., Ziegler-Ulsh, C., and Douds, D. 2010. How to inoculate arbuscular mycorrhizal fungi on the farm, part 1. Rodale Institute. https://rodaleinstitute.org/science/articles/how-to-innoculate-arbuscular-mycorrhizal-fungi-on-the-farm-part-1/
2 Salomon, M.J., Demarmels, R., Watts-Williams, S.J., et al. 2022. Global evaluation of commercial arbuscular mycorrhizal inoculants under greenhouse and field conditions. Applied Soil Ecology. 169: 104225. https://doi.org/10.1016/j.apsoil.2021.104225
3 Siemering, G., Ruark, M., Arriaga, F., Silva, E., and Johnson, H. 2016. The value of arbuscular mycorrhizal fungi for field crops. University of Wisconsin Extension. A4114-01. https://learningstore.extension.wisc.edu/products/the-value-of-arbuscular-mycorrhizal-fungi-for-field-crops-p1799
4 Stahl, L., Fernandez, F., and Kaiser, D. 2018. Reduce the risk of fallow syndrome with cover crops. University of Minnesota Extension. https://extension.umn.edu/cover-crops/reduce-risk-fallow-syndrome-cover-crops#protecting-nitrogen-investments-735160
1210_400801
Disclaimer
Always read and follow pesticide label directions, insect resistance management requirements (where applicable), and grain marketing and all other stewardship practices.