Effect of Soil Saturation on Available Nitrogen
May 15, 2025
- When soils become saturated from heavy rainfall, loss of nitrogen (N) becomes a major concern.
- After soils are saturated, the two processes that can reduce the amount of available N are denitrification (microbial conversion of nitrate to nitrogen gases) and leaching.
- Estimating N loss is not an exact science; however, some guidelines exist that can help with N assessment.
Loss of Nitrogen
Denitrification is a process through which soil bacteria convert nitrate-N (NO3-) to N gas (N2) that then escapes from the soil into the air. Denitrification occurs under anaerobic soil conditions, meaning conditions in which there is no oxygen in the soil. Because of this, nitrogen loss can occur rapidly if soils are saturated or flooded as the water fills all available gaps between the soil particles, forcing air out. Studies conducted in Illinois showed that up to 5% nitrate-N loss occurred through denitrification each day soils were saturated.1
Once anaerobic conditions occur, the rate of denitrification is influenced by the chemical form the nitrogen is in and the temperature. Nitrogen in the ammonium-N form (NH4+) is not subject to denitrification, but nitrate-N (NO3-) is. Urea converts to nitrate—and thus becomes subject to denitrification—about twice as quickly as anhydrous ammonia does.2 In soils where saturation or ponding typically occurs, special consideration should be made to either not apply N until the risk of soil saturation decreases or to only apply N as ammonium (NH4+) until the crop is ready to use nitrate (NO3-).
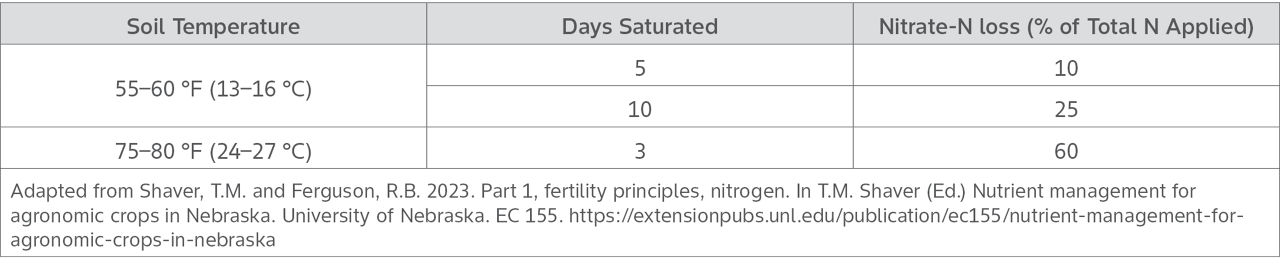
The rate of denitrification also increases as soil temperatures increase above 50 °F (10 °C). University of Nebraska data (Table 1) demonstrates the potential nitrate-N loss for every day of saturation at various temperatures.3
Table 1. Estimated denitrification losses influenced by soil temperature and days of saturation.
In addition to denitrification, saturated soils can also lose N through leaching. Leaching is more of a concern in soils that allow rapid downward movement of water such as sandy soils, well drained soils, and/or soils with improved drainage.4 Ammonium nitrate and urea ammonium nitrate (UAN) solutions are more susceptible to leaching than anhydrous ammonia. However, once any form of fertilizer N is converted to nitrate by soil bacteria, it will experience the same amount of denitrification and leaching, regardless of the original N source in the fertilizer.
Is Supplemental Nitrogen Necessary?
Applications of supplemental N may be warranted if sufficient loss has occurred. The amount of N loss is hard to quantify as it depends on several factors, including soil type/structure, soil temperature, form of N, and days of saturation. One method to determine if supplemental N is required is the pre-sidedress nitrogen test (PSNT). PSNT soil samples should be collected to a depth of 1 foot when corn is between 6 and 12 inches (15 and 30 cm) tall. The accuracy of the test is highly dependent upon the sampling and handling procedures. Contact your lab for proper sampling and handling techniques. Test results of over 25 ppm may indicate that no additional N will be needed for the growing season. Test results less than 25 ppm can indicate you may get a positive yield response from sidedressed N. In theory, the lower the test, level the more N you will need to apply. Refer to local university guidelines or consult an agronomist for the amount of additional N to apply if PSNT results are under 25 ppm.
Estimating Nitrogen Loss
- Calculate N present as nitrate: Start with the pounds of N applied per acre and multiply that by the percent (as a decimal) of the N in the fertilizer that was in nitrate form.
- Calculate N denitrified: Use the pounds of nitrate/acre from Step 1 and multiply that by the percent denitrification from Table 1, above, that best matches the conditions in the field. This calculation is only intended as a rough estimate.
Note that the conversion to nitrate occurs almost immediately with N applied as urea. With 28% or 32% UAN, half of the N is in the urea form, 25% is found as ammonium, and the remaining 25% as nitrate. The nitrate is already subject to loss, and the other fractions are readily converted. Conversion of N applied as anhydrous ammonia is delayed 10 to 14 days following application, regardless of any stabilizer added. Soil temperature has a large influence on the conversion of ammonium to nitrate. It takes approximately 2 weeks for complete conversion at 60 °F and 1 week is needed at 70 °F.2
Nitrogen Management
Nitrogen should be applied via sidedressing if a significant amount of N has been lost through denitrification or leaching. UAN liquid solutions can be applied as a band on the surface with drop nozzles, even on fairly large corn. To help minimize volatilization and maximize effectiveness, rainfall or irrigation is needed to move UAN and urea into the soil. Up to 30% of the urea could be lost due to volatilization if no rainfall occurs within two weeks and temperatures are warm.5
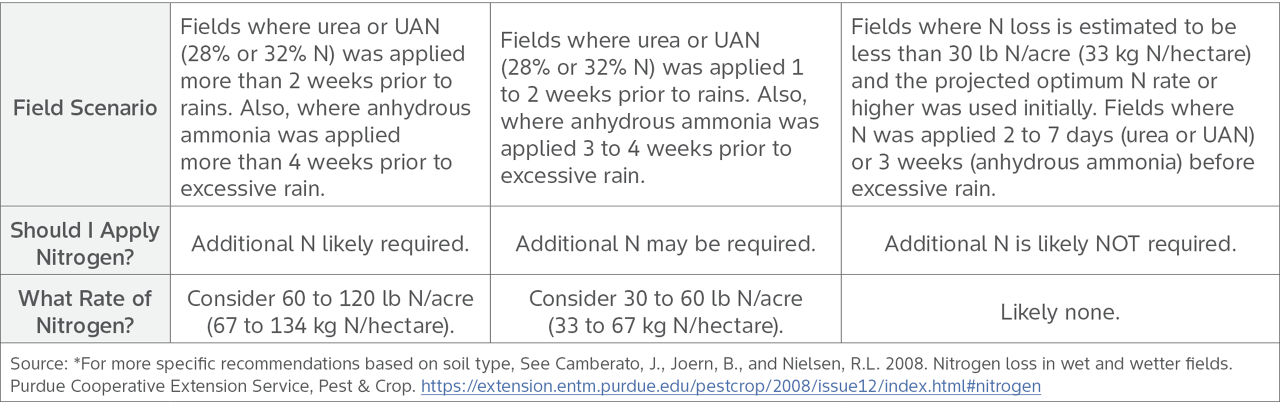
To help optimize yield potential in corn, adequate N is critical. Purdue University provides general recommendations by N form, timing of N application prior to excessive rain, and soil type (Table 2).5 Assessing N loss and requirements is not an exact science, but doing so can help provide estimates that impact your bottom line.
Table 2. Estimated nitrogen applications to replace lost nitrogen, based on nitrogen form and timing of nitrogen prior to excessive rain.*
Related Articles
Corn Management in Flooded Fields
Sources
1Sawyer, J. 2008. Estimating nitrogen losses. Iowa State University Extension and Outreach, Integrated Crop Management. https://crops.extension.iastate.edu/cropnews/2008/06/estimating-nitrogen-losses
2Fernandez, F.G. and D.E. Kaiser. 2021. Understanding nitrogen in soils. University of Minnesota. https://extension.umn.edu/nitrogen/understanding-nitrogen-soils
3Shaver, T.M. and Ferguson, R.B. 2023. Part 1, fertility principles, nitrogen. In T.M. Shaver (Ed.) Nutrient management for agronomic crops in Nebraska. University of Nebraska. EC 155. https://extensionpubs.unl.edu/publication/ec155/nutrient-management-for-agronomic-crops-in-nebraska
4Elgie, C. 2023. Nitrogen losses in saturated soils. Ontario Ministry of Agriculture, Food and Rural Affairs, Field Crop News. https://fieldcropnews.com/2023/07/nitrogen-losses-in-saturated-soils/
5Camberato, J., Joern, B., and Nielsen, R.L. 2008. Nitrogen loss in wet and wetter fields. Purdue Cooperative Extension Service, Pest & Crop. https://extension.entm.purdue.edu/pestcrop/2008/issue12/index.html#nitrogen
1213_120982
Disclaimer
Always read and follow pesticide label directions, insect resistance management requirements (where applicable), and grain marketing and all other stewardship practices.