10 MIN READ
Nitrogen Availability in the Spring
January 17, 2025
- Nitrogen (N) is available from many sources and in many forms, not all of which are useful to plants.
- Spring N availability is determined by N losses and gains, which are influence by many factors such as soil type, weather conditions, microbial activity, the type of fertilizer used, the specific nutrient compound applied, and whether nitrogen stabilizers were used.
- Determining the amount of N available for plant uptake in the spring and planning nitrogen management steps needed in-season can help maximize crop yield potential.
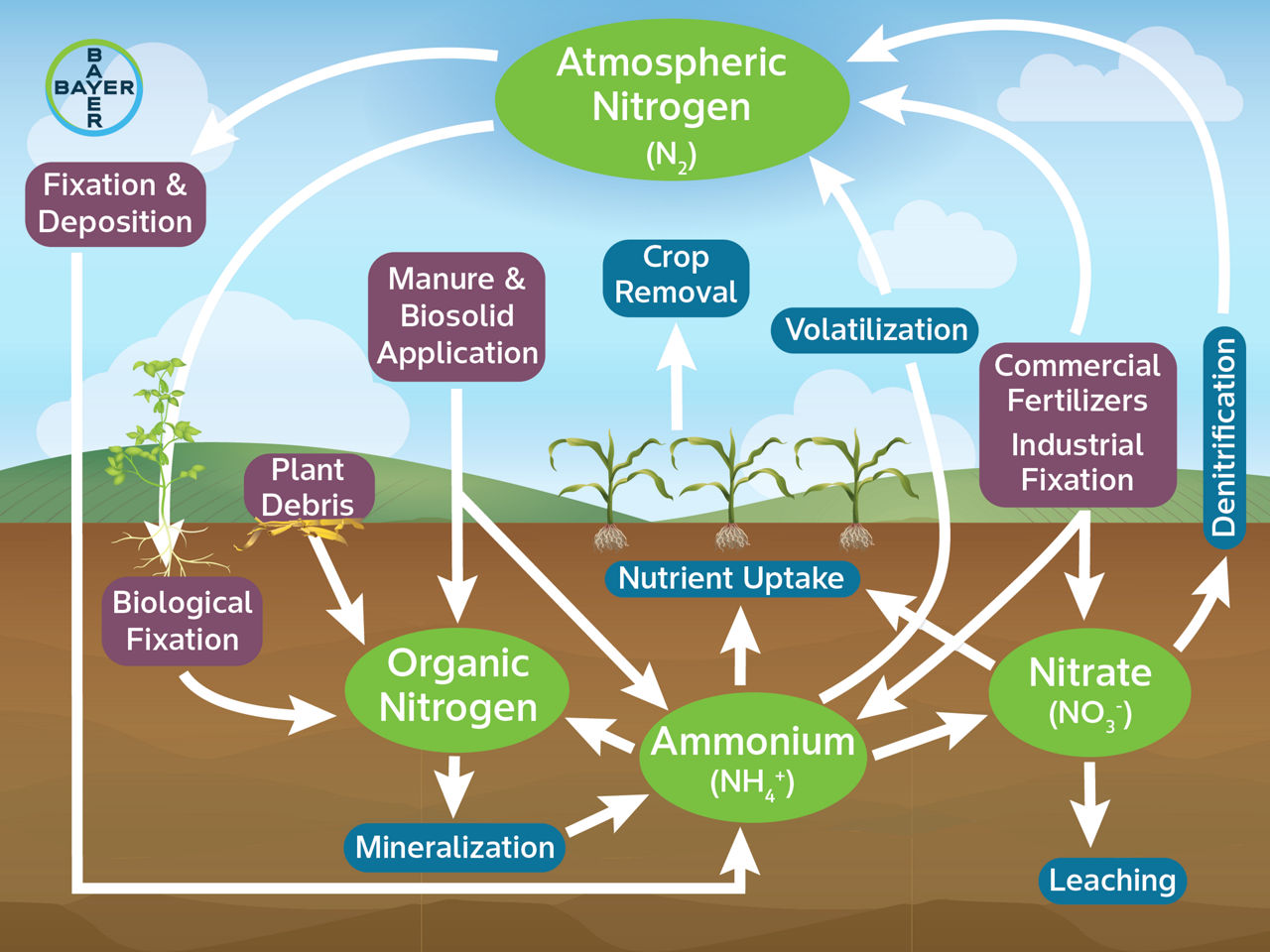
Sources of Nitrogen
Nitrogen (N) exists in many chemical forms in the environment, and all N changes between these chemical forms through a global process known as the nitrogen cycle. All plants require nitrogen to grow. However, not every form of nitrogen is chemically available to plants, because plants are only able to break down and absorb certain forms of nitrogen. Available nitrogen can be supplied for plant growth in several ways.
Atmospheric nitrogen (N2) is a major component of the nitrogen cycle but is not available to plants. Legumes, such as soybean and alfalfa, have symbiotic relationships with specific bacteria in their roots that can absorb N2 from the air through a process called nitrogen fixation.1,2 Once the symbiotic bacteria absorb the N2, they break it down into ammonia (NH3), a form of nitrogen that plants can use.2 Some of this ammonia is taken up by the legume and some is released into the soil. Small amounts of atmospheric N2 are also added to the soil through precipitation.1
Mineralization occurs when certain soil microorganisms convert the several organic forms of N that are unavailable to plants to ammonium (NH4), which is plant available.3 Soil organic matter is an important source of N for plant growth. Decomposition of this organic matter happens slowly, releasing about 20 pounds of N per acre (22 kilograms of N per hectare) per year for each percent of organic matter content in the soil.1
Commercial N fertilizers can also be applied as a primary source of N to enhance plant growth, increase yields, and sustain profits. These products come in various forms, discussed later in this article.
How Nitrogen is Lost from Soil
Denitrification is the process by which soil bacteria convert nitrate (NO3) and nitrite (NO2) into nitrogen gases (N2 and N2O) that are then lost to the atmosphere.4 Most denitrification occurs when soils are saturated, and the amount of nitrate lost is influenced primarily by the duration of the saturation and by soil temperature. University of Illinois estimates indicate that when soils are saturated, denitrification can cause a daily loss of one to two percent of soil nitrate if soil temperatures are less than 55 °F (13 °C), a two to three percent loss if soil temperatures are between 55 and 65 °F (13 and 18 °C), and a four to five percent loss if soil temperatures above 65 °F (18 °C).5
Leaching occurs in saturated soils when water moves nitrate (NO3) downward in the soil profile and out of the root zone. In sandy soils, leaching may cause nitrates to contaminate groundwater but in heavier-textured soils, leached nitrates typically reach tile lines and may eventually contaminate surface water.
Volatilization can occur when urea-based fertilizers (or other sources of ammonia, NH3) are surface-applied and not incorporated. Urease enzymes in soil and plant residue convert urea to free ammonia gas.6,7 Volatilization is promoted by excessive plant debris on the soil surface, by warm windy days, and by a soil pH value greater than 7.0.7 To minimize N loss by volatilization, incorporation should occur within three to four days after application with tillage, a half-inch of rain or irrigation, or with the use of a urease inhibitor.8 Under warm, sunny conditions, up to 20% of urea-based N can volatilize within a week of application.9
Nitrogen Fertilizers
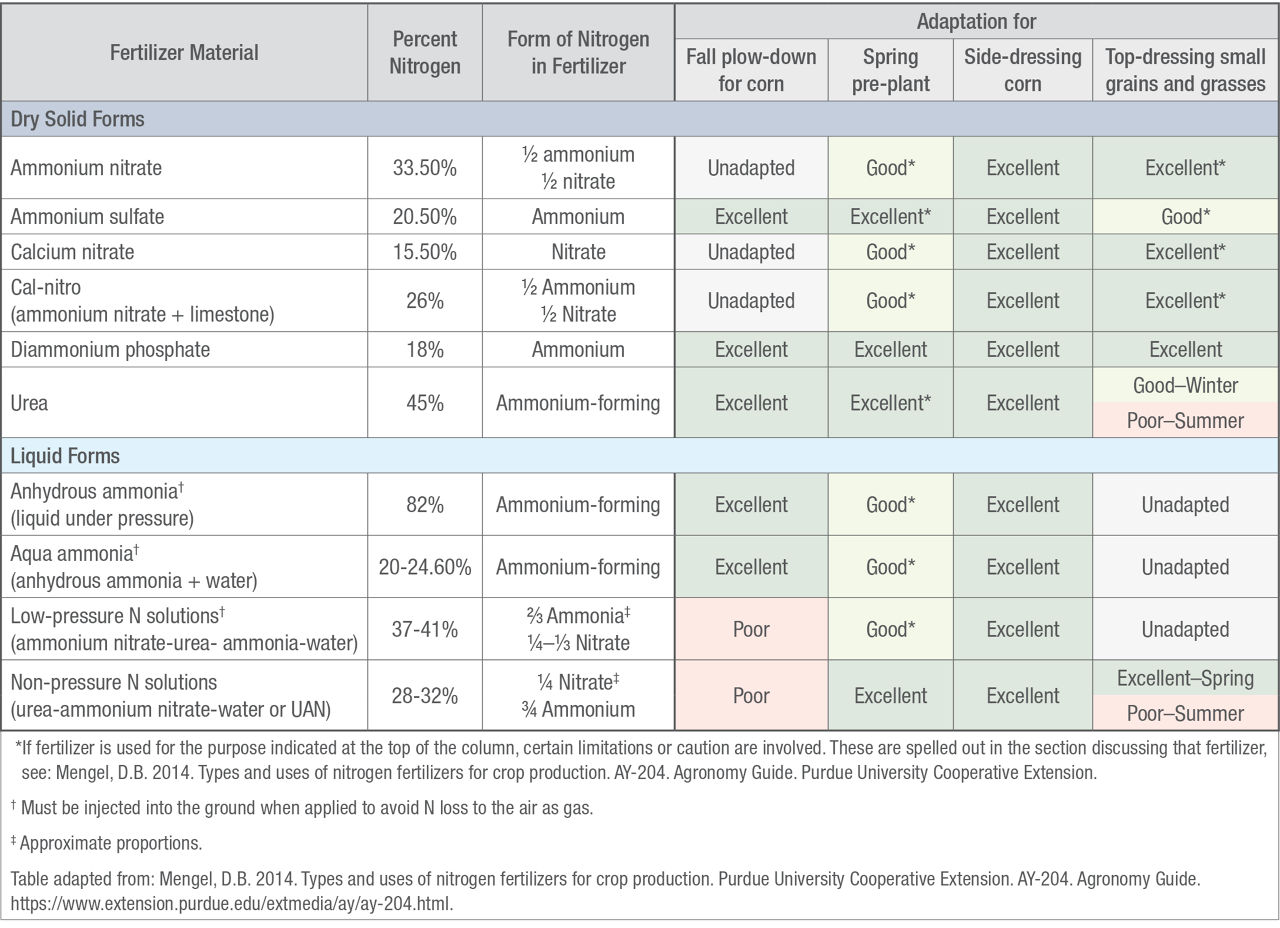
Nitrogen fertilizers contain N in one or more forms: ammonia (NH3), ammonium (NH4), nitrate (NO3), or urea (CO(NH2)2) (Table 1). Anhydrous ammonia is a compressed gas injected into the soil to minimize N fertilizer loss and can be applied in the fall, spring, or sidedressed. When anhydrous ammonia is applied, the ammonia is converted to ammonium in the soil almost immediately. Ammonium binds to clay and organic matter in the soil, preventing it from leaching. Through nitrification, soil microorganisms convert ammonium to nitrate, the primary form of N that can be taken up by plants. Conditions favorable to nitrification are a soil pH of 7.0, soil moisture at 50% of water holding capacity, and a soil temperature of about 80 °F (27 °C).7 Applying anhydrous ammonia to soils that are too wet or too dry can result in loss of N to the atmosphere due to poor sealing of the knife track. To help reduce seed and seedling injury, planting should be delayed three to five days after application of anhydrous ammonia. For preplant applications, consider applying anhydrous ammonia at an angle to your rows to help minimize seedling injury by minimizing direct contact.
When soil moisture and temperatures are favorable, urea is converted to ammonia two to four days after application. Lower temperatures can slow the process. Urea is first converted to ammonia, then to ammonium, and finally to nitrate, which is readily available to plants but at risk for leaching or denitrification. No matter what form of N fertilizer is used, most of the N will be converted to nitrate in the soil.
Table 1. Characteristics and adaptation of common nitrogen fertilizers.
Nitrogen Stabilizers
Nitrogen additives can be used to delay N transformations and prolong N availability. If nitrogen stabilizers are used with N fertilizer applications, nitrogen loss may be reduced even if weather conditions are not ideal.
There are two categories of N stabilizers in common use: nitrification inhibitors and urease inhibitors. Nitrification inhibitors inhibit soil bacteria responsible for denitrification, slowing the conversion of ammonium to nitrate. Urease inhibitors can treat urea to reduce N loss to ammonia volatilization.10 Additional nitrogen stabilizers can include polymer-coated urea for slow release of N and products that contain urea, a urease inhibitor, and a nitrification inhibitor.11
For more specific information on nitrogen and corn, please read this article: Benefits of Nitrogen for Corn Production.
Determining Nitrogen Availability Using Soil Tests
Determining how much nitrogen is available in fields can be challenging, because N availability is influenced by the type of N fertilizer applied, the timing and method of application, soil type, previous crop, use of nitrogen stabilizers, and weather conditions, among other factors. There are several tests for nitrogen content to help growers determine the N availability in their fields. Which tests are most useful will depend on the unique conditions in each field.
Nitrate and ammonium soil testing can help estimate the amount of N loss due to rainfall or flooding. To conduct these tests, soil cores should be collected to a depth of at least one foot. In sandy soils prone to leaching, sampling at a greater depth may help to identify plant-available N deeper in the soil profile.12 If fertilizer was broadcast in the fall or early spring, collect 20 to 30 cores per sample. If previously applied fertilizer was banded, samples should contain 15 to 20 soil cores. Samples should be collected perpendicular to the direction in which fertilizer was applied. Each sample should represent no more than 10 acres.9 Samples should be dried or refrigerated as soon as possible to stop soil microbial activity from changing N levels and thus reducing the accuracy of the test. Results indicating substantial levels of soil ammonium are more likely if anhydrous ammonia was recently applied, nitrogen stabilizers were used, or soil pH is 5.5 or less. In such cases, low levels of soil nitrate may mean that little conversion of ammonium to nitrate occurred rather than that nitrate was lost from the soil due to leaching or denitrification.
A Preplant Soil Nitrate Test (PPNT) can provide helpful information where corn follows corn in fields in which dry weather has reduced N loss. This test can be used in early spring to adjust the total amount of spring-applied N by the amount indicated in the soil test.13 Since samples are collected in early spring, the procedure measures mostly N carried over from the previous crop and any N applied in the previous fall season. Heavy rainfall in late spring or after testing, a previous crop of legumes, or a manure application the previous summer or fall may reduce the usefulness of this test.13
A Pre-Sidedress Nitrate Test (PSNT) is useful for fields where manure or other organic fertilizers have been applied recently or where legume crops—such as alfalfa—have been grown. Growers may also want to consider using this test for fields that had fall-applied N and suspected N loss from leaching and denitrification. The test provides a measure of the amount of N mineralized from organic N plus the amount of carryover N still present in the soil.5 However, if late spring temperatures are below normal, the test may underestimate soil N as lower temperatures cause slower mineralization rates. Sampling for the PSNT should be done when corn is 6 to 12 inches (15 to 30 cm) tall or from late May to early June. Soil cores should be taken at a depth of one foot, with one sample containing 15 to 20 cores. Although some differences exist in university recommendations for interpreting PSNT results, a general rule of thumb is that if soil test results are over 23 to 25 ppm, additional nitrogen is probably not needed.5,14
The following articles are available for more information on soil testing: Soil Testing 101 and Reading and Interpreting a Soil Test.
In-Season Nitrogen Estimation
There are also other available tools for detecting nitrogen deficiencies in season, such as chlorophyll leaf meters (SPAD meters), crop sensors, and remote sensing/aerial imagery.5 Remote sensing requires good canopy development mid-season and can be accomplished effectively using NDVI (normalized difference vegetative index) cameras on aerial drones or sensors fitted to high clearance N application equipment. Remote sensing tests work by comparing the NDVI or NDRE (normalized difference red edge) readings for a given part of the field to the areas with the highest readings in the field, which may be small reference areas that were deliberately created using extra N fertilizer applications. These comparisons then allow growers to provide variable-rate N adjustments to areas of the field that appear N deficient.15 Any of these tools may provide growers with more information to aid in determining late-season nitrogen application rates.
For more information on estimating in-season nitrogen sufficiency, please see the article from University of Nebraska-Lincoln: https://cropwatch.unl.edu/2020/tips-season-nitrogen-management-corn.
Sources
1Fernandez, F.G. and Kaiser, D.E. 2021. Understanding nitrogen in soils. University of Minnesota Extension. https://extension.umn.edu/nitrogen/understanding-nitrogen-soils#immobilization-761163
2Clay, D.E. and Gustafson, K. 2020. 23: Nitrogen fixation. In, Best management practices for soybean production. South Dakota State University Extension. https://extension.sdstate.edu/igrow-soybeans-best-management-practices-soybean-production
3Brune, D., Killpack, S.C., and Buchholz, D. 2022. Nitrogen in the environment: Mineralization –immobilization. University of Missouri Extension. Publication No. WQ260. https://extension.missouri.edu/publications/wq260
4Brune, D., Killpack, S.C., and Buchholz, D. 2022. Nitrogen in the environment: Denitrification. University of Missouri Extension. Publication No. WQ255. https://extension.missouri.edu/publications/wq255
5Nafziger, E. 2021. Nitrogen management for corn. In, Illinois Agronomy Handbook. https://extension.missouri.edu/publications/wq255
6Brune, D., Killpack, S.C., and Buchholz, D. 2022. Nitrogen in the environment: Ammonia volatilization. University of Missouri Extension. Publication No. WQ257. https://extension.missouri.edu/publications/wq257
7Mengel, D.B. Types and uses of nitrogen fertilizers for crop production. Purdue University Cooperative Extension, Agronomy Guide. AY-204. https://www.extension.purdue.edu/extmedia/ay/ay-204.html
8Scharf, P., Lory, J., and Grundler, J. 2018. Best management practices for nitrogen fertilizer in Missouri. University of Missouri Extension. Publication No. IPM1027. https://extension.missouri.edu/publications/ipm1027
9Camberato, J., and Nielsen, R.L. 2017. Soil sampling to assess current soil N availability. Purdue University Extension, The Chat’n Chew Café. https://www.agry.purdue.edu/ext/corn/news/timeless/AssessAvailableN.html
10Ferguson, R., Maharjan, B., Wortmann, C., and Krienke, B. 2019. Nitrogen inhibitors for improved fertilizer use efficiency. University of Nebraska-Lincoln, CropWatch. https://cropwatch.unl.edu/2019/nitrogen-inhibitors-improved-fertilizer-use-efficiency
11Franzen, D.W. 2022. Nitrogen extenders and additives for field crops. North Dakota State University Extension Service. SF1581. https://www.ag.ndsu.edu/publications/crops/nitrogen-extenders-and-additives-for-field-crops
12Fernandez, F.G., Hoeft, R.G., and Randall, G.W. 2011. How much nitrogen is there in the spring from fall-applied MAP, DAP, and ammonium sulfate? Proc. of the 2011 Wisconsin Crop Management Conference. 50:127-135. https://soilsextension.qa.webhosting.cals.wisc.edu/wp-content/uploads/sites/68/2016/07/Fernandez_map.pdf
132021. Soil nitrate tests for corn production in Wisconsin. University of Wisconsin–Madison Extension, Nutrient and Pest Management Program. https://ipcm.wisc.edu/wp-content/uploads/sites/54/2022/11/UWSoilNitrateTests_final.pdf
14Shapiro, C., Hergert, G., and Ferguson, R. 2012. Using the PSNT for spring testing of nitrogen availability. University of Nebraska–Lincoln, CropWatch. https://cropwatch.unl.edu/using-psnt-spring-testing-nitrogen-availability-unl-cropwatch-may-23-2012
15Iqbal, J., Wortmann, C., Maharjan, B., and Puntel, L. 2020. Tips for in-season nitrogen management in corn. University of Nebraska–Lincoln, CropWatch. https://cropwatch.unl.edu/2020/tips-season-nitrogen-management-corn
Web sources verified 10/10/24. 1213_103578
Disclaimer
Always read and follow pesticide label directions, insect resistance management requirements (where applicable), and grain marketing and all other stewardship practices.