5 MIN READ
Cotton Nematode Predictive Sampling and Prevention
September 18, 2024
Nematodes are microscopic roundworms found throughout cotton-growing areas and beyond. There are four major nematode species that attack cotton: root-knot (Meloidogyne spp.), reniform (Rotylenchulus reniformis), lance (Hoplolaimus spp.), and sting (Belonolaimus longicaudatus) nematodes. Root-knot and reniform nematodes cause the most damage to cotton.1 Indeed, nematode infestations cause yield loss and millions of dollars of revenue loss. From 2020 through 2023, estimated yield losses due to root-knot nematodes averaged 2.5% per year. During that same time period, yield losses due to reniform nematodes were estimated to average nearly 1% per year.1 Yield losses from nematode damage are often attributed to poor soil conditions, nutrient deficiency, disease, or other environmental conditions. Nematode damage limits nutrient and moisture uptake by plants and predisposes them to infection by fungal and bacterial pathogens.
Nematodes can complete their lifecycle (egg, four juvenile stages, adult) in one month or less, producing several generations during a growing season. Mature female root-knot and reniform nematodes can produce hundreds of eggs, leading to rapid population growth.2
For more information about identifying cotton nematodes, please refer to Nematodes in Cotton. https://www.cropscience.bayer.us/articles/dad/nematode-management-in-cotton.
Predictive Sampling
Predictive soil sampling is essential for developing a nematode management plan. Cotton nematodes spend their entire life in the soil and are classified by where they live while feeding on the plant. Endoparasitic nematodes, like root-knot nematodes, live within the root structure, while ectoparasitic nematodes, such as sting nematodes, live in the soil outside of the root.2 It is important to understand where nematodes live when sampling for them.

Nematode populations are typically highest around harvest, so sampling is recommended either within one month before harvest, immediately after harvest, or after the last irrigation. Samples should be taken with adequate soil moisture, though sample immediately if low soil moisture conditions persist two weeks after harvest. Collect soil cores with a standard soil probe at a 45° angle, six to 12 inches deep, and no more than four to five inches away from the planted row (Figure 1). Targeting the root zone ensures the inclusion of root fragments, increasing the likelihood of collecting endoparasitic nematodes in the sample.3 A sample should consist of at least 20 individual cores that are collected in a random pattern, mixed together, and packaged according to laboratory directions. The area represented by a sample should be limited to 20 acres or less (10 acres is preferred) and segregated by soil type, drainage, and crop history. Store samples out of direct light and at room temperature. Send samples to the diagnostic lab soon after sampling (within a week), ideally at the beginning of the week to ensure that the sample arrives before the weekend.4
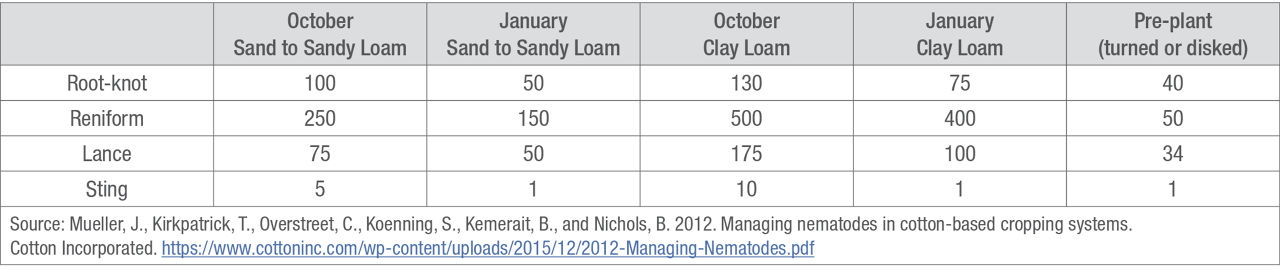
Lab results will estimate the risk of nematode problems for the following year’s crop. Understanding the nematode species present and their population levels can help growers determine if a management plan should be started, as can the general threshold levels for the sampling time (Table 1).4 Thresholds can vary by state or region. Consult with university or local experts for threshold values specific to your geographic area.
Table 1. General damage thresholds (number of nematodes per 100 cm3 soil) as affected by sampling date, soil type, and tillage.
Planning for Prevention
Host Avoidance
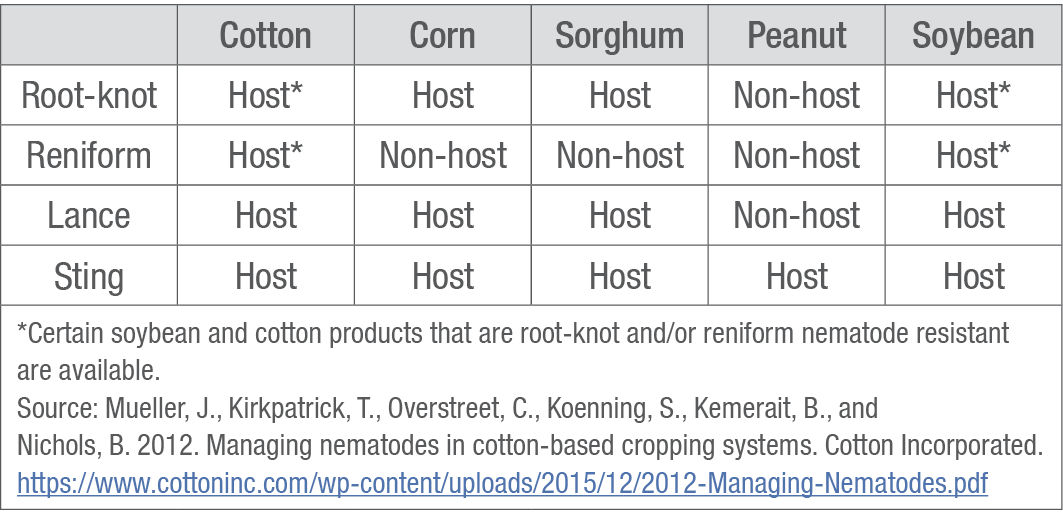
Rotation. Rotate to non-host crops to interrupt the nematode lifecycle and reduce populations (Table 2). Peanut is not a host for root-knot, reniform, or lance nematodes. Corn is not a host for reniform nematodes, and soybean can be an option if nematode-resistant soybean products are planted.
Table 2. Nematode hosts and non-hosts in a cotton cropping system.
Weed management. Maintain post-harvest weed management. Many common weed species are also nematode hosts, including:
- Root-knot nematode: nutsedge, select pigweed, horseweed (marestail), teaweed, select morningglory, tall ironweed, black nightshade, and common Bermudagrass.
- Reniform nematode: sicklepod, Carolina geranium, morningglory, pigweed species, and purslane.
- Lance nematode: morningglory and pigweeds.
- Sting nematode: nutsedge and ragweed.4
Product Selection
Cotton seed product selection. If root-knot or reniform nematodes are present in a field, cotton products with nematode resistance (NR) can provide an effective way to help reduce damage.5 Four Deltapine® brand cotton products with NR have been developed for fields that are moderately to highly infested with root-knot and/or reniform nematodes. Several products contain the native breeding trait that can resist root-knot or reniform nematodes, while others have a stack of two NR traits that provide protection against both root-knot and reniform nematodes. Nematode resistance traits can also help reduce the population of these two species compared to planting a susceptible product. Deltapine® cotton seed products with NR have been bred for high yield potential even in fields that are not infested with root-knot and/or reniform nematodes.
Nematicides. There are several options available for growers to consider and use prior to and at planting.
- Seed treatments. Acceleron® Solutions Offering ELITE plus Copeo® Seed Treatment provides protection against damage from a wide range of nematode species.
- Fumigants. Cotton growers with heavy nematode pressure may combine seed treatment nematicides with fumigants such as Telone® II applied preplant.6 Chemical application timing and additional safety precautions may limit flexibility during the growing season.
- In furrow, at planting. Velum® fungicide can be applied either in-furrow or through a chemigation system; see label for further information or instructions. AgLogic™15G Aldicarb Pesticide is a granular product that may help to reduce insect and nematode damage.6 This product is not registered for use in all states and may not be available in your state. AgLogic 15G and Telone II are both Restricted Use Pesticides.
Sources
1Lawrence, K., Faske, T., Hagan, A., et al. 2024. Cotton disease loss calculator. In, Estimates of crop yield losses due to diseases and invertebrate pests: An online tool. Crop Protection Network. https://loss.cropprotectionnetwork.org/crops/cotton-diseases?year_start=2020&year_end=2023&diseaseCategory=19&diseases%5B%5D=150&country=1®ion=&cropID=5
2Plant parasitic nematodes. University of Florida. https://mrec.ifas.ufl.edu/lso/SCOUT/Nematodes.htm
3Soil sampling to assess cotton nematode population distribution and densities. National Cotton Council of America. https://www.cotton.org/tech/pest/nematode/soil.cfm
4Mueller, J., Kirkpatrick, T., Overstreet, C., Koenning, S., Kemerait, B., and Nichols, B. 2012. Managing nematodes in cotton-based cropping systems. Cotton Incorporated. https://www.cottoninc.com/wp-content/uploads/2015/12/2012-Managing-Nematodes.pdf.
5Dudak, J., Noland, R., Isakeit, T., Wheeler, T., McKnight B., and Morgan, G. 2021. Reniform nematodes in cotton – new genetic resistance offers relief. Texas A&M AgriLife Extension, Texas Row Crops Newsletter. https://agrilife.org/texasrowcrops/2021/06/02/reniform-nematodes-in-cotton-new-genetic-resistance-offers-relief/
6Jones, M.A., Farmaha, B.S., Greene, J., Marshall, M., Mueller, J.D., and Smith, N.B. Rev. 2023. South Carolina cotton growers’ guide. Clemson University Cooperative Extension Service. EC 589. https://www.clemson.edu/extension/agronomy/resources/request-handbook.html
Web sources verified 08/21/24. 1411_69479
Disclaimer
Always read and follow pesticide label directions, insect resistance management requirements (where applicable), and grain marketing and all other stewardship practices.